Back
- Home
- About Us
Pro
10 day free trial. No credit card required.$75 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- -
- -
- -
Team
18 day free trial. No credit card required.$150 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- -
- -
Agency
30 day free trial. No credit card required.$400 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- Multi-Rater Review
- -
Best ValueEnterprise
+1-750-0616
Consulting Time 8am - 8pm
Custom business solution trailered to your needs
- Recruit (Custom)
- Payroll (Custom)
- Expense (Custom)
- Cliq (Custom)
- Vault (Custom)
- Assessments
- Our Products
Implant
Pro
10 day free trial. No credit card required.$75 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- -
- -
- -
Instrument
Team
18 day free trial. No credit card required.$150 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- -
- -
External Fixator
Agency
30 day free trial. No credit card required.$400 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- Multi-Rater Review
- -
Best ValueExternal Fixator
Agency
30 day free trial. No credit card required.$400 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- Multi-Rater Review
- -
Best Value - We Serve
- Resources
- Contact
Search
Close this search box
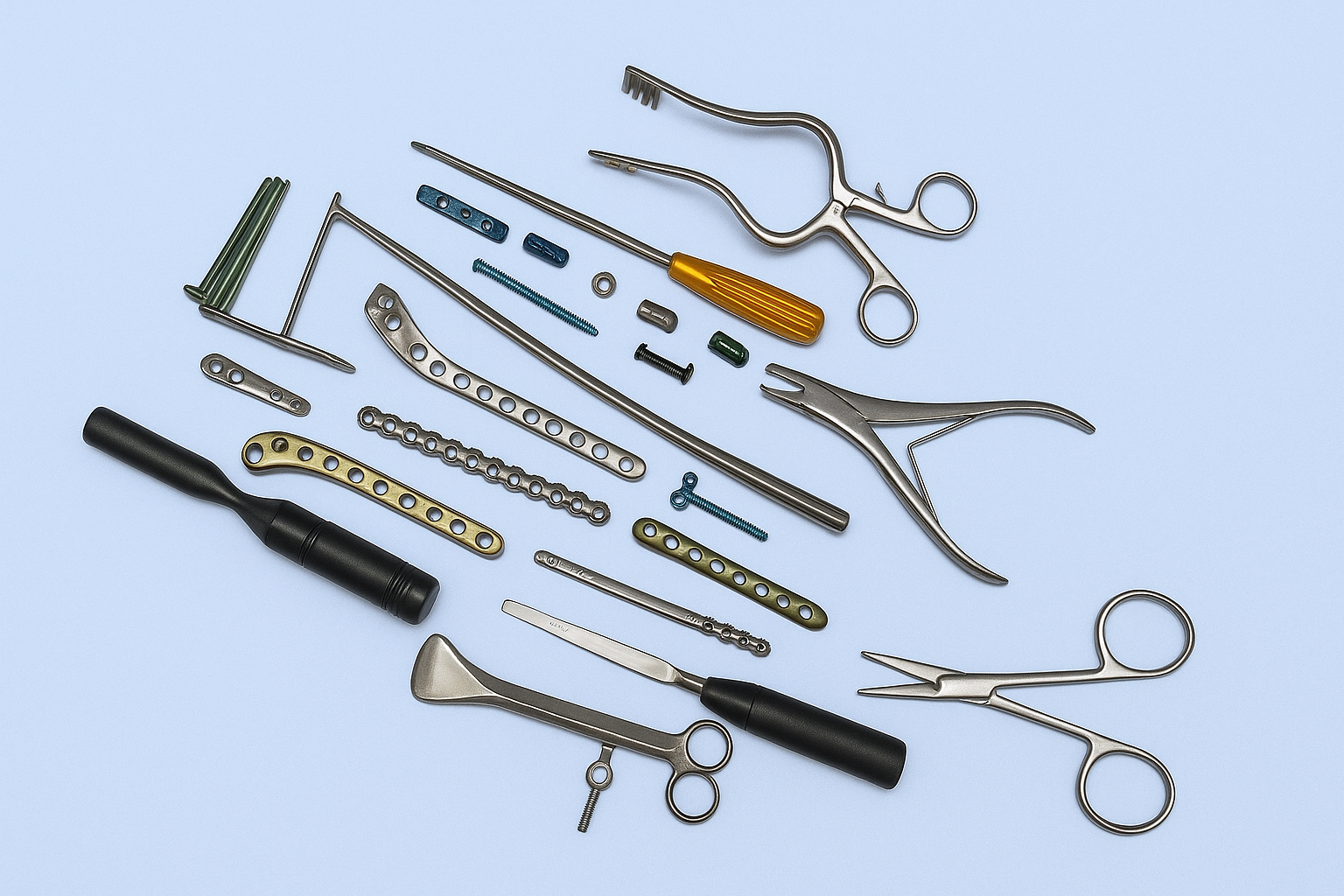
The Importance of Quality Standards in Orthopaedic Implant Manufacturing
Orthopaedic implants are life-changing medical devices that restore mobility, reduce pain, and improve the quality of life for millions of patients worldwide. But the success of these implants depends largely on one critical factor: quality standards. In an industry where precision, durability, and patient safety are paramount, following stringent international standards is not optional—it is essential.
This blog highlights the importance of quality standards in orthopaedic implant manufacturing and why hospitals, surgeons, and patients should prioritize them.
Why Quality Standards Matter in Orthopaedic Implants
Why Quality Standards Matter in Orthopaedic Implants
01
The foremost reason behind strict standards is patient safety. Orthopaedic implants must withstand years of stress and function seamlessly inside the human body. Any compromise in quality can lead to complications, revision surgeries, or even life-threatening risks. Certified implants ensure biocompatibility, durability, and safe integration with bone and tissue.
02
Surgeons rely on implants to perform critical procedures successfully. When implants are manufactured under ISO, CE, and FDA-certified standards, surgeons gain confidence in their reliability. Hospitals also prefer vendors who meet these certifications, knowing it reduces risk for both patients and practitioners.
03
With orthopaedic implants being exported across 30+ countries, manufacturers must comply with global regulatory frameworks. Standards like ISO 13485, CE Marking, and US FDA approval ensure that the products are accepted internationally, making them trustworthy and legally marketable.
04
Inferior quality implants can fail prematurely, leading to painful and costly revision surgeries. Standard-compliant implants undergo rigorous testing for mechanical strength, fatigue resistance, and material quality—significantly lowering the chances of implant failure and improving long-term outcomes.
05
Adherence to quality standards doesn’t just ensure safety; it also drives innovation. Manufacturers invest in R&D, advanced materials, and precision technology to stay ahead of compliance requirements. This results in better implant designs, minimally invasive solutions, and faster patient recovery.
06
- ISO 13485: Global standard for medical device quality management systems.
- CE Certification: Ensures compliance with European Union health, safety, and environmental requirements.
- FDA Approval (US): Certifies safety and effectiveness for implants sold in the United States.
- • WHO-GMP: Good Manufacturing Practices ensuring consistency and quality across production processes.
07
At Uma Surgicals, quality is the foundation of everything we do. With state-of-the-art manufacturing, advanced machinery, and strict quality protocols, we deliver orthopaedic implants that comply with global standards and certifications. Our dedication ensures that surgeons and hospitals can trust our implants for safe, reliable, and effective patient outcomes.
In orthopaedic implant manufacturing, quality standards are not just benchmarks—they are the backbone of trust, safety, and global recognition. Choosing certified implants means choosing patient well-being, surgeon confidence, and long-term success.
Mumbai
Corporate Office
102/103, Auto Commerce
House, Kennedy Bridge,
Nana Chowk,
Mumbai – 400 007, India.
House, Kennedy Bridge,
Nana Chowk,
Mumbai – 400 007, India.
Company
We build really better idea
Subscribe for newsletter & get day news, service updates
Useful Links
Follow Us
Palghar
Manufacturing Unit
Plot no 49,kidc,
village poman, taluka
vasai , district palghar,
India – 401208
village poman, taluka
vasai , district palghar,
India – 401208
© 2025 Uma surgicals Pvt Ltd All Rights Reserved
