- Home
- About Us
Pro
10 day free trial. No credit card required.$75 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- -
- -
- -
Team
18 day free trial. No credit card required.$150 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- -
- -
Agency
30 day free trial. No credit card required.$400 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- Multi-Rater Review
- -
Best ValueEnterprise
+1-750-0616
Consulting Time 8am - 8pm
Custom business solution trailered to your needs
- Recruit (Custom)
- Payroll (Custom)
- Expense (Custom)
- Cliq (Custom)
- Vault (Custom)
- Assessments
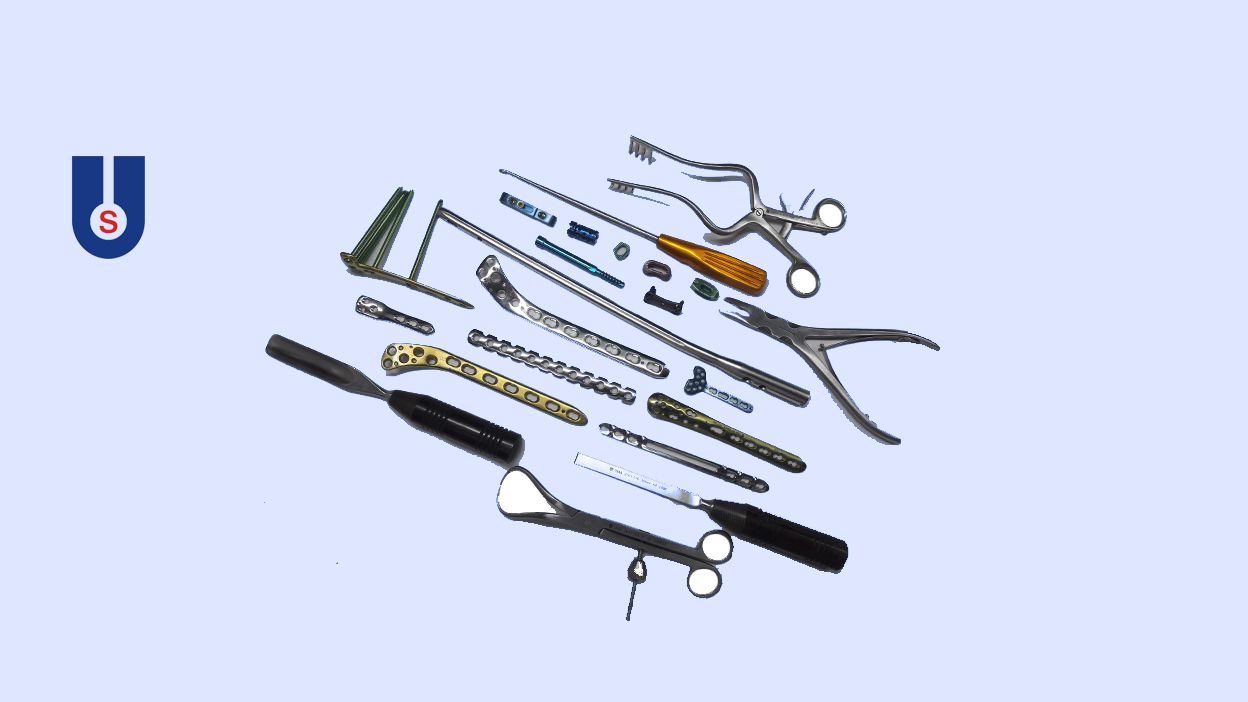
- Our Products
Implant
Pro
10 day free trial. No credit card required.$75 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- -
- -
- -
Instrument
Team
18 day free trial. No credit card required.$150 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- -
- -
External Fixator
Agency
30 day free trial. No credit card required.$400 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- Multi-Rater Review
- -
Best ValueExternal Fixator
Agency
30 day free trial. No credit card required.$400 USD Billed Monthly- Timesheet Scheduler
- People Kiosk
- Shift Scheduling
- Shift Rotation
- Multi-Rater Review
- -
Best Value - We Serve
- Resources
- Contact
About us
Uma Surgicals Pvt Ltd – Innovating Healthcare Since 1980
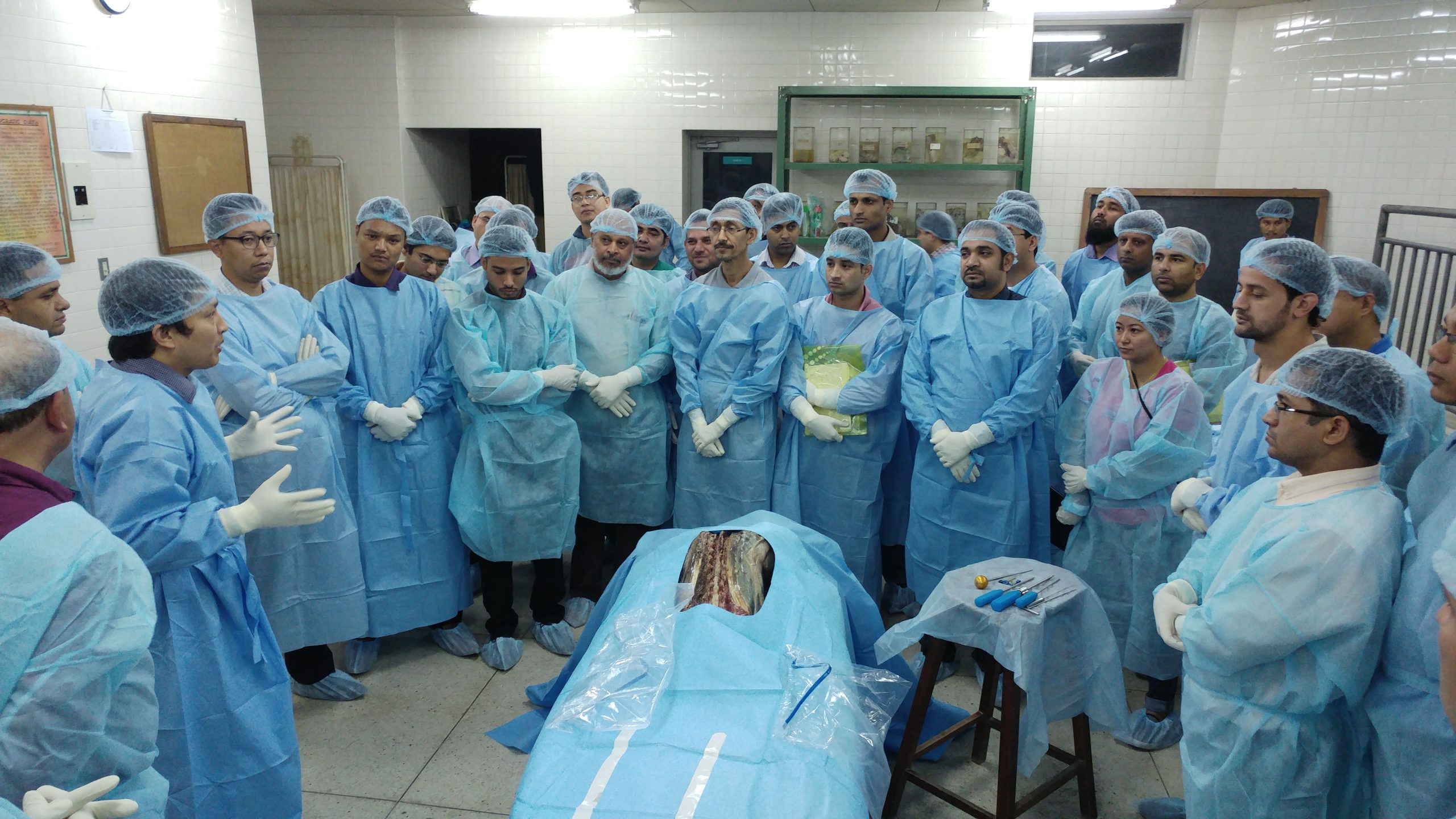
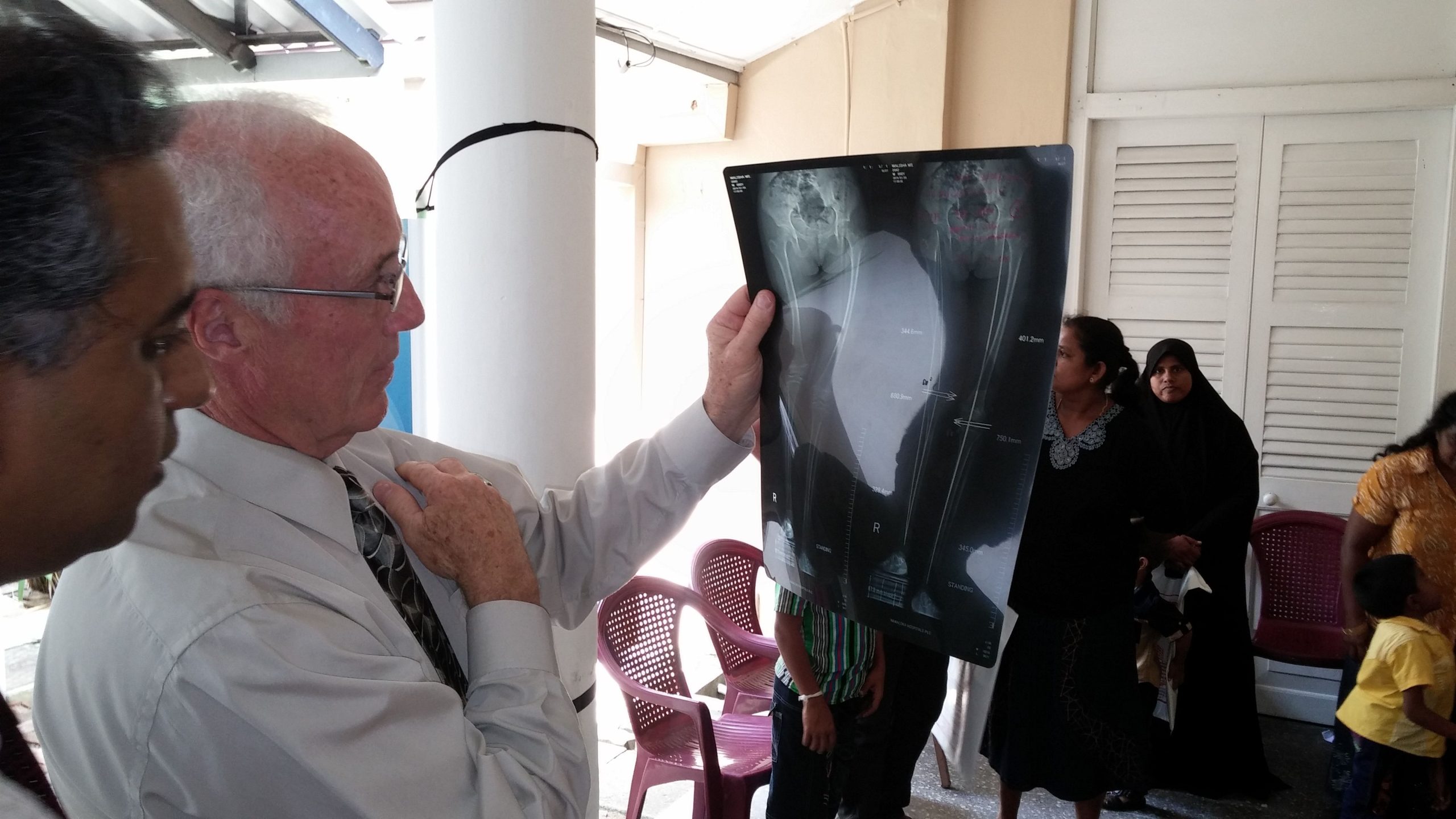
We take pride in our active contribution to the medical community, having participated in over 360 global conferences and conducted 120+ live surgery workshops and demonstrations till date.

Founded in 1980 in a small workshop in South Mumbai, Uma Surgicals Pvt Ltd has grown into one of India’s most trusted and pioneering manufacturers of Orthopaedic (Trauma), Spine and Maxillofacial Implants, Surgical Instruments, and General Surgery Instruments.
For over four decades, we have been at the forefront of innovation in the medical device industry, consistently adapting the latest technologies and manufacturing practices to deliver safe, reliable, and cost-effective solutions for healthcare professionals worldwide.

Commitment, Excellence, Integrity: Our Core Beliefs
Our Vision
Our Mission
Our Core Values
• Innovation – Driving continuous improvements in healthcare technologies.
• Integrity – Building trust through ethical business practices and transparency.
• Quality – Ensuring precision, safety, and compliance in every product.
• Affordability – Making world-class surgical solutions accessible to all.
• Collaboration – Working hand-in-hand with surgeons, hospitals, and institutions globally.
• Excellence – Setting benchmarks in product design, manufacturing, and service delivery.
Proudly Recognized by the International College of Surgeons (US Section) and
the American Academy of Neurological & Orthopaedic Surgeons.

Our Core Values
Driving continuous improvements in healthcare technologies.
Building trust through ethical business practices and transparency.
Ensuring precision, safety, and compliance in every product.
Making world-class surgical solutions accessible to all.
Working hand-in-hand with surgeons, hospitals, and institutions globally.
Setting benchmarks in product design, manufacturing, and service delivery.

Company story
We work for better growing result step-by-step
1980

Foundation in South Mumbai
Trade stocks of the biggest names in the international stock market
1990s

Pioneer of Spine Implants manufacturing in India
Trade stocks of the biggest names in the international stock market
2000s

Pioneer of Paediatric Implants manufacturing in India
Trade stocks of the biggest names in the international stock market
2010s

Participation in over 400 conferences and 150+ workshops, with Global presence over 30 Countries
Trade stocks of the biggest names in the international stock market
2020

A new US FDA compliant manufacturing facility with Global presence over 40+ countries
Trade stocks of the biggest names in the international stock market
Why Choose Us
Our team
Meet the Team

MR. Vijay P. Gangan
B.E. (Civil Engg)
Founder and Director

Mr. Girish V. Gangan
M.S. (Indl Engg, USA), B.E. (Prod. Engg, Mumbai)
Managing Director and CEO

Mr. Shirish V. Gangan
B.Sc., Diploma in Business Management
Sales Director

Mrs. Kavita V. Gangan
M.Sc.(Bio Chemistry), B. Sc
UMA
Co-Author of "Third Dimension of Surgery" Book published by CBS Publications.


Testimonials
Clients feedback



Our Manufacturing Process
Raw Material Selection
1. High-grade medical stainless steel, titanium, and cobalt-chromium alloys are sourced from certified suppliers.
2. Every batch undergoes chemical and mechanical testing to meet ISO, CE, and FDA standards.
Precision Forging & CNC Machining
1. CNC (Computer Numerical Control) machines ensure consistent accuracy in dimensions.
2. Forging enhances the strength and durability of orthopedic implants.
3. Complex geometries for spine and maxillofacial implants are shaped using multi-axis machining centers.
Heat Treatment & Hardening
1. Implants are heat-treated in controlled furnaces to achieve the required hardness and tensile strength.
2. This ensures longer life, higher wear resistance, and load-bearing capacity.
Surface Treatment & Polishing
1. Surgical implants undergo electropolishing, passivation, and anodizing (for titanium).
2. This improves corrosion resistance, biocompatibility, and smooth finish, making implants safe for long-term use inside the body.
3. Instruments are mirror-polished for ease of sterilization and longer usability.
Quality Control & Testing
1. Each product undergoes dimensional accuracy checks with CMM (Coordinate Measuring Machines).
2. Mechanical strength testing ensures implants can withstand real-life surgical stresses.
3. Biocompatibility and corrosion tests guarantee patient safety.
4. 100% inspection is carried out before packaging.
Cleaning & Sterilization
1. Ultrasonic cleaning removes microscopic impurities.
2. Final sterilization is done in a controlled cleanroom environment.
Packaging & Labelling
1. Products are packed in sterile, tamper-proof packaging to maintain safety until they reach surgeons.
2. Every pack is barcoded/QR coded for traceability and compliance with regulatory requirements.
Regulatory Compliance & Certifications
1.Manufacturing follows ISO 13485, CE, and WHO-GMP standards.
2. Documentation is maintained for traceability, audits, and export compliance.
Continuous Improvement
1. Regular feedback is collected from surgeons, hospitals, and distributors.
2. Our R&D team collaborates with healthcare professionals to co-develop new implants and instruments, ensuring Uma Surgicals stays ahead in innovation.
Workshop
Enhancing Skills Through Surgical Workshops
Trust &
Accreditation
Our Certifications







Mumbai
Corporate Office
House, Kennedy Bridge,
Nana Chowk,
Mumbai – 400 007, India.
Company
We build really better idea
Subscribe for newsletter & get day news, service updates
Useful Links
Follow Us
Palghar
Manufacturing Unit
village poman, taluka
vasai , district palghar,
India – 401208